What is Corneal Dystrophy?
Corneal dystrophies are a group of genetic, often progressive, eye disorders in which abnormal material often accumulates in the clear (transparent) outer layer of the eye (cornea). Corneal dystrophies may not cause symptoms (asymptomatic) in some individuals; in others they may cause significant vision impairment. The age of onset and specific symptoms vary among the different forms of corneal dystrophy. The disorders have some similar characteristics; most forms of corneal dystrophy affect both eyes (bilateral), progress slowly, do not affect other areas of the body, and tend to run in families. Most forms are inherited as autosomal dominant traits; a few are inherited as autosomal recessive traits.
An international classification of the corneal dystrophies has been developed that takes into account the chromosomal loci of the various corneal dystrophies as well as the responsible genes and their mutations. Traditionally, these disorders have been classified based upon their clinical findings and the specific layer of the cornea affected. Advances in molecular genetics (e.g., identification of specific disease genes) have led to a greater understanding of these disorders.
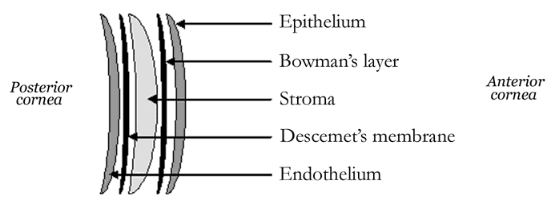
The cornea is made up of five distinct layers:
- The Epithelium – the outermost, protective layer of the cornea.
- The Bowman’s membrane – this second layer is extremely tough and difficult to penetrate further protecting the eye
- The Stroma – the thickest layer of the cornea, consisting of water, collagen fibers and other connective tissue components that give the cornea its strength, elasticity and clarity.
- Descemet’s Layer – a thin, strong inner layer that also acts as a protective layer.
- The Endothelium – the innermost layer consisting of specialized cells that pump excess water out of the cornea
Corneal Dystrophy Symptoms
The symptoms of corneal dystrophies result from the accumulation of abnormal material within the cornea, the clear outer layer of the eye. The cornea serves two functions; it protects the rest of the eye from dust, germs and other harmful or irritating material, and it acts as the eye’s outermost lens, bending incoming light onto the inner lens, where the light is then directed to the retina (a membranous layer of light-sensing cells in the back of the eye). The retina converts light to images, which are then transmitted to the brain. The cornea must remain clear (transparent) to be able to focus incoming light.
Corneal dystrophies are characterized by the accumulation of foreign material in one or more of the five layers of the cornea. Such material may cause the cornea to lose its transparency potentially causing loss of vision or blurred vision.
A symptom common to many forms of corneal dystrophy is recurrent corneal erosion, in which the outermost layer of the cornea (epithelium) does not stick (adhere) to the eye properly. Recurrent corneal erosion can cause discomfort or severe pain, an abnormal sensitivity to light (photophobia), the sensation of a foreign body (such as dirt or an eyelash) in the eye, and blurred vision.
Standard Therapies
Diagnosis
The presence of a corneal dystrophy may be found incidentally during a routine eye examination. A diagnosis may be confirmed by a thorough clinical evaluation, a detailed patient history and a variety of tests, such as a slit lamp examination, in which a special microscope (slit lamp) allows a physician to view the eye through high magnification. Some specific corneal dystrophies can be diagnosed with molecular genetic tests even before symptoms develop.
Treatment
The treatment of corneal dystrophies varies. Individuals who do not have symptoms (asymptomatic) or only have mild symptoms may not require treatment and may instead be regularly observed to detect potential progression of the disease.
Specific treatments for corneal dystrophies may include eye drops, ointments, lasers and corneal transplant. Recurrent corneal erosions (a common finding in most corneal dystrophies) may be treated with lubricating eye drops, ointments, antibiotics or specialized (bandage soft) contact lenses. If recurrent erosions persist, additional measures such as corneal scraping or the use of excimer laser therapy, which can remove abnormalities from the surface of the cornea (phototherapeutic keratectomy).
In individuals with significant associated symptoms a corneal transplant, known as a keratoplasty, may be necessary. Corneal transplants have been highly successful in treating individuals with advanced symptoms of corneal dystrophies. There is a risk however, in certain corneal dystrophies, that the lesions will eventually develop in the graft (donated) cornea.
Types of Corneal Dystrophies
- Anterior Corneal Dystrophies
- Epithelial Basement Membrane Dystrophy (EBMD, ABMD, Map Dot)
- Lisch Corneal Dystrophy
- Meesmann Corneal Dystrophy
- Reis-Buckler Corneal Dystrophy
- Thiel-Behnke Corneal Dystrophy
- Stromal Corneal Dystrophies
- Gelatinous Droplike Corneal Dystrophy
- Granular Corneal Dystrophy Type I
- Granular Corneal Dystrophy Type II (Avellino corneal dystrophy)
- Lattice Corneal Dystrophy Type I
- Lattice Corneal Dystrophy Type II
- Macular Corneal Dystrophy
- Posterior Corneal Dystrophies
- Congenital Hereditary Endothelial Corneal Dystrophy
- Fuchs Endothelial Corneal Dystrophy
- Posterior Polymorphous Corneal Dystrophy
- Schnyder Crystalline Corneal Dystrophy
Anterior Corneal Dystrophies
These corneal dystrophies affect the outer layers of the cornea including the epithelium, the epithelial basement membrane (a thin membrane that separates epithelial cells from underlying tissue), and the Bowman membrane.
Epithelial Basement Membrane Dystrophy
This form of corneal dystrophy is characterized by the development of very tiny dots (microcysts), gray areas that, collectively, resemble the outlines of countries on a map, or fine lines that resemble fingerprints on the epithelial layer of the cornea. Most individuals do not have any symptoms (asymptomatic). In some cases, symptoms may include recurrent erosions and blurred vision, which affect apparently 10 percent of individuals. An abnormal sensitivity to light (photophobia) and the sensation of foreign material within the eye may also occur. Epithelial basement membrane dystrophy is a common form of corneal dystrophy and is also known as map-dot-fingerprint dystrophy and Cogan microcystic dystrophy.
Lisch Corneal Dystrophy
This rare form of corneal dystrophy is characterized by clusters of multiple, tiny cysts or lesions that may be band-shaped or curved or spiraled (whorled) in appearance. In some cases, affected individuals do not have any symptoms (asymptomatic). Some individuals may have decreased clarity of vision (visual acuity), blurred vision, and double vision affecting only one eye (monocular diplopia).
Meesmann Corneal Dystrophy
This extremely rare form of corneal dystrophy affects the epithelial layer of the cornea. It is characterized by the development of clusters of multiple, small, clear cysts. The cysts are roughly the same size. Affected individuals may experience mild irritation and a slight decrease in clarity of vision (visual acuity). A sensitivity to light (photophobia) and excessive tear formation (lacrimation) can occur in this form of corneal dystrophy. Clouding (opacity) of the cornea rarely occurs, but may develop in some elderly individuals. Meesmann corneal dystrophy is also known as juvenile epithelial dystrophy.
Reis-Buckler Corneal Dystrophy
This form affects the Bowman membrane and is characterized by clouding (opacity) and progressive scarring of the membrane. During the first decade of life, affected individuals may initially develop recurrent erosions that cause significant pain. Recurrent erosions may eventually stabilize as affected individuals grow older. Additional symptoms may develop including an abnormal sensitivity to light (photophobia), a feeling or sensation of a foreign body in the eye, and a marked decrease in clarity of vision (visual acuity) often by 20 years of age. Reis-Buckler corneal dystrophy is also known as granular corneal dystrophy type III or corneal dystrophy of Bowman layer type I.
Thiel-Behnke Corneal Dystrophy
This form of corneal dystrophy affects the Bowman membrane and may be extremely difficult to distinguish from Reis-Buckler corneal dystrophy. The abnormalities affecting the cornea may resemble honeycombs. Recurrent corneal erosions begin during childhood, but visual acuity is not affected until later during life. Pain and an abnormal sensitivity to light (photophobia) may also occur. Thiel-Behnke corneal dystrophy is also known as honeycomb corneal dystrophy or corneal dystrophy of Bowman layer type II.
Stromal Corneal Dystrophies
These corneal dystrophies affect the stromal or central layer of the cornea. Some of these disorders can progress to affect other layers of the cornea.
Gelatinous Droplike Corneal Dystrophy
Also known as familial subepithelial corneal dystrophy, develops in individuals during the first decade of life and is characterized by loss of vision, an abnormal sensitivity to light (photophobia), excessive tearing (lacrimation), and the feeling (sensation) of foreign substances in the eye. Gelatinous masses of amyloid, a type of protein, accumulate beneath the corneal epithelium and make the cornea opaque and progressively impair vision.
Granular Corneal Dystrophy Type I
This form of corneal dystrophy is characterized by the development of small particles (granules) that collectively resemble breadcrumbs, usually during the second or third decade of life. These lesions slowly grow eventually combining (coalescing) to form larger lesions. Individuals may develop recurrent erosions. Although vision is usually unaffected early in the disease, decreased visual acuity may occur by the fourth or fifth decade. Some individuals may have an abnormal sensitivity to light (photophobia). Eye pain may result from recurrent corneal erosions.
Granular Corneal Dystrophy Type Ii
In granular corneal dystrophy type II, also known as Avellino corneal dystrophy, lesions develop on the stroma usually beginning in the first or second decade of life. The opacities in the cornea sometimes resemble a cross between the granular lesions of granular corneal dystrophy type 1 and lattice lesions of lattice corneal dystrophy (see below). As affected individuals age, the lesions may become larger, more prominent and involved the entire stromal layer. Some older individuals have decreased clarity of vision (visual acuity) due to haze (clouding of the cornea). Recurrent erosions may develop in some cases.
Lattice Corneal Dystrophy
Lattice corneal dystrophies are a common form of stromal dystrophy and two main variants have been identified. They are characterized by the development of lesions that form branching lines that resemble cracked glass or the crisscrossed, overlapping pattern of lattice. Lattice dystrophy type I and its variants usually occur by the end of the first decade. Recurrent corneal erosions (which can be painful) often precede these characteristic changes to the stroma. Affected individuals may have decreased clarity of vision (visual acuity) and an abnormal sensitivity to light (photophobia).
Lattice Dystrophy Type II
Is classified as a corneal dystrophy, but occurs as part of a larger disorder called Merejota syndrome, which is more serious than the corneal disease.
Macular Corneal Dystrophy
Individuals with this form of corneal dystrophy are born with clear corneas, but eventually develop clouding of the stroma, usually between 3-9 years of age. Progression of the lesions results in decreased clarity of vision (visual acuity) and irritation early during life. In some cases, significant vision loss can occur by the second decade. Severe vision loss may develop by the third or fourth decade. Painful recurrent erosions sometimes occur, but are less common than in other corneal dystrophies affecting the stroma. Macular corneal dystrophy is also known as Groenouw dystrophy type II.
Schnyder Crystalline Corneal Dystrophy
This form of corneal dystrophy usually develops during the second decade of life, but can develop as early as the first year of life. Affected individuals develop opaque corneas due to an accumulation of fat or cholesterol within the stroma that eventually cause clouding, haziness and blurred vision. Crystals commonly accumulate in the cornea. Affected individuals have visual impairment that is worsened by glare.
Posterior Corneal Dystrophies
These corneal dystrophies affect the innermost layers of the cornea including Descemet membrane and the endothelium, which are the layers of the cornea closest to the inner structures of the eye. These disorders can potentially progress to affect all layers of the cornea.
Congenital Hereditary Corneal Dystrophy
Two types of congenital hereditary corneal dystrophy exist:
Type 1, inherited as an autosomal dominant trait, is characterized by swelling (edema) of the cornea, pain, and corneas that are clear at birth, but become cloudy during early infancy.
Type II, inherited as an autosomal recessive trait, is characterized by corneal swelling and cloudy corneas at birth. Rapid, jittery eye movements (nystagmus) may occur with this form.
Type II, the recessive form, is more common than the dominant form.
Fuchs’ Endothelial Dystrophy
This form of corneal dystrophy usually develops during middle age, although there may be no symptoms initially (asymptomatic). Fuchs dystrophy is characterized by problems with tiny cells called “pumper” cells on the innermost layer of the cornea. Normally, these cells pump water out of the eye. In Fuchs dystrophy these cells deteriorate (“die off”) and the cornea fills with water and swells. The swelling worsens and blurred vision occurs that is worse in the morning, but gradually improves throughout the day. Tiny blisters form on the cornea, eventually rupturing and causing extreme pain. Affected individuals may also have a gritty or sandy feeling within the eye (foreign body sensation), be abnormally sensitive to light and see a glare or halo when looking at lights. As the disease progresses, vision no longer improves during the day and significant vision loss may occur, possibly necessitating a corneal transplant.
Posterior Polymorphous Dystrophy
This uncommon form of corneal dystrophy may present at birth (with clouding of the cornea) or later during life and is characterized by lesions affecting the endothelium. Most individuals do not develop symptoms (asymptomatic). Effects on the cornea may be slowly progressive. Both eyes are usually affected, but one eye may be more severely affected than the other (asymmetric). In severe cases, individuals with posterior polymorphous dystrophy may develop swelling (edema) of the stroma, an abnormal sensitivity to light (photophobia), decreased vision, and the feeling (sensation) of foreign material in the eye. In rare cases, increased pressure with the eye (intraocular pressure) may occur.
Causes – Genetics
Most cases of corneal dystrophy are inherited as an autosomal dominant trait with variable expressivity. Genetic diseases are determined by the combination of genes for a particular trait that are on the chromosomes received from the father and the mother.
Dominant genetic disorders occur when only a single copy of an abnormal gene is necessary for the appearance of the disease. The abnormal gene can be inherited from either parent, or can be the result of a new mutation (gene change) in the affected individual. The risk of passing the abnormal gene from affected parent to offspring is 50 percent for each pregnancy regardless of the sex of the resulting child.
Variable expressivity means that some individuals who inherit the same gene for a dominant disorder may not develop (express) the same symptoms.
The epithelial basement membrane, Reis-Buckler, Thiel-Behnke, Meesmann, Schnyder, lattice type I, lattice type II, granular type I, granular type II (Avellino), congenital hereditary corneal dystrophy type I, and posterior polymorphous forms of corneal dystrophy have autosomal dominance inheritance. Fuchs dystrophy may have autosomal dominant inheritance in some cases; in others it may occur spontaneously for no apparent reason (sporadic). Macular corneal dystrophy and congenital hereditary corneal dystrophy type II forms of corneal dystrophy have autosomal recessive inheritance.
Recessive genetic disorders occur when an individual inherits the same abnormal gene for the same trait from each parent. If an individual receives one normal gene and one gene for the disease, the person will be a carrier for the disease, but usually will not show symptoms. The risk for two carrier parents to both pass the defective gene and, therefore, have an affected child is 25 percent with each pregnancy. The risk to have a child who is a carrier like the parents is 50 percent with each pregnancy. The chance for a child to receive normal genes from both parents and be genetically normal for that particular trait is 25 percent. The risk is the same for males and females.
Investigators have determined that several corneal dystrophies occur due to disruptions or changes (mutations) of the transforming growth factor beta-induced (TGFB1) gene located on the long arm (q) of chromosome 5 (5q31). Chromosomes, which are present in the nucleus of human cells, carry the genetic information for each individual. Human body cells normally have 46 chromosomes. Pairs of human chromosomes are numbered from 1 through 22 and the sex chromosomes are designated X and Y. Males have one X and one Y chromosome and females have two X chromosomes. Each chromosome has a short arm designated “p” and a long arm designated “q”. Chromosomes are further sub-divided into many bands that are numbered. For example, “chromosome 5q31” refers to band 31 on the long arm of chromosome 5. The numbered bands specify the location of the thousands of genes that are present on each chromosome.
A variety of epithelial basement, Reis-Buckler, Thiel-Behnke, granular types I and II, and lattice types I corneal dystrophies have all been linked to the transforming growth factor beta induced (TGFB1) gene. These forms of corneal dystrophy develop due to different mutations of this gene, which was formerly known as the beta-induced gene human cell clone number 3 (BIGH3) gene. The TGFB1 gene contains instructions for creating (encoding) a protein known as transforming growth factor beta induced protein (keratoepithelin), which aids the corneal layers to remain stuck (adhered) together. An accumulation of this protein due to a mutated gene causes the symptoms of the corneal dystrophies associated with this gene.
Meesmann corneal dystrophy has been linked to mutations to two separate genes, one (KTR3) on the long arm of chromosome 17 (17q12) and one (KTR12) on the long arm of chromosome 12 (12q13). These genes contain instructions for creating (encoding) certain proteins called keratins essential for the proper formation of the cornea.
Some cases of Thiel-Behnke corneal dystrophy have been linked to mutations of a gene located on the long arm of chromosome 10 (10q23-q24).
Macular corneal dystrophy has been linked to mutations of the carbohydrate sulfotransferase-6 (CHST6) gene on the long arm of chromosome 16 (16q22). This gene encodes for keratan sulfate, a complex sulfated carbohydrate that is essential for the proper development of cartilage and the cornea. There is no creation (synthesis) of normal keratan sulfate.
Schnyder corneal dystrophy has been linked to mutations of the UBIAD1 gene located on the short arm of chromosome 1 (1p34-q36).
Posterior polymorphous dystrophy has been linked to three different chromosomes. One is on the long arm of chromosome 20 (20p11.2), another is on chromosome 1 (1p34.3-p32.3) involving the COL8A2 gene, and a third is due to a mutation in the TCF8 gene on chromosome 10 (10p11-q11).
The autosomal recessive form of congenital hereditary endothelial corneal dystrophy is due to mutations in the SLC4A11 gene on chromosome 20(20p13). The gene for autosomal dominant congenital hereditary endothelial corneal dystrophy has not been identified, but it is located on the short arm of chromosome 20 (20p11.2-q11.20).
Lisch corneal dystrophy has been linked to a gene on the short arm of the X chromosome (Xp23). It is believed that this form of corneal dystrophy is inherited as an X-linked dominant trait. X-linked dominant disorders are caused by an abnormal gene on the X chromosome. Males with an abnormal gene are more severely affected than females.
Affected Populations
Corneal dystrophies affect women and men in equal numbers, except for Fuchs corneal dystrophy which affects women about four times as often as men. The corneal dystrophies can affect individuals of any age. The incidence of corneal dystrophies is unknown. Because some individuals do not have symptoms (asymptomatic), determining the true frequency of these disorders in the general population is difficult.
Related Disorders
Symptoms of the following disorders can be similar to those of corneal dystrophy. Comparisons may be useful for a differential diagnosis.
Keratoconus is a noninflammatory eye (ocular) condition characterized by progressive changes of the shape of the cornea. The cornea is the thin-walled, “dome-shaped” transparent layer forming the front of the eyeball; it serves as a protective covering and helps to focus or bend (refract) light waves onto the retina at the back of the eye. In individuals with keratoconus, slowly progressive thinning of the cornea causes it to bulge or protrude forward in an irregular, cone-like (conical shape) leading to blurry vision, an increased sensitivity to light, and other vision problems. Keratoconus often begins at puberty. Although the specific underlying cause of the condition is unknown, investigators indicate that genetic factors may play some role. In addition, in some cases, keratoconus may occur in association with a variety of other disorders. (For more information on this disorder, choose “keratoconus” as your search term in the Rare Disease Database.)
Bullous keratopathy is an eye condition characterized by swelling (edema) of the cornea due to abnormal water retention by the cornea. Bullous keratopathy can result in pain and loss of vision. Small blisters (bullae) can form on the surface of the eye, which can potentially rupture causing severe pain and infection. Bullous keratopathy can be caused by surgery to the eye, trauma to the eye, and inflammatory eye disorders.
Factors
Several factors determine what therapies may be used to treat individuals with corneal dystrophies including the specific type of corneal dystrophy present, the severity of associated symptoms, the rate of progression of the disease, and a patient’s overall health and quality of life.
Genetic counseling may be of benefit for affected individuals and their families. Other treatment is symptomatic and supportive.
Investigational Therapies
Information on current clinical trials is posted on the Internet at www.clinicaltrials.gov. All studies receiving U.S. Government funding, and some supported by private industry, are posted on this government web site. For information about clinical trials being conducted at the NIH Clinical Center in Bethesda, MD, contact the NIH Patient Recruitment Office:
Toll free: (800) 411-1222
TTY: (866) 411-1010
Email: prpl@cc.nih.gov
For information about clinical trials sponsored by private sources, contact:
www.centerwatch.com
Other Organizations
Other helpful resources and organizations related to Corneal Dystrophies
Avellino Lab USA, Inc.
1505 Adams Drive, Suite B-2
Menlo Park CA 94025
Phone#: 650-396-3741
www.avellinolab.com/
Eye Bank Association of America
1015 18th Street, NW
Washington DC 20036
Phone #: 2027754999
info@restoresite.org
www.restoresight.org
Genetic and Rare Diseases (GARD) Information Center
PO Box 8126
Gaithersburg MD 20898-8126
Phone #: (888)820-52311
rarediseases.info.nih.gov/GARD
National Organization for Rare Disorders
55 Kenosia Avenue
Danbury, CT 06810
Phone #: (800) 999-6673
www.rarediseases.org
NIH/National Eye Institute
31 Center Dr
Bethesda MD 20892-2510
Phone #: 3014965248
2020@nei.nih.gov
www.nei.nih.gov
The availability of genetic analyses has demonstrated the shortcomings of the current phenotypic method of corneal dystrophy classification. Abnormalities in different genes can cause a single phenotype, whereas different defects in a single gene can cause different phenotypes. Some disorders termed corneal dystrophies do not appear to have a genetic basis.
The Corneal Dystrophy Foundation is grateful to the National Organization of Rare Diseases for giving us permission to publish this information and to Gordon K. Klintworth, MD, PhD, Professor of Pathology at Duke University Medical Center and Joseph A.C. Wadsworth, MD, Research Professor of Ophthalmology, Duke University Medical Center.
This information is for educational purposes only. It should never be used for diagnostic or treatment purposes. If you have questions regarding a medical condition, always seek the advice of your physician or other qualified health professional. Our booklets and brochures provide a brief overview of rare diseases. For more specific information, we encourage you to contact your personal physician.